
Have you ever wondered what temperature does aluminium melt at or why this property is so important in industries and even in everyday products? The melting temperature of aluminium is more than just a number—it's a key to understanding how this versatile metal is shaped, joined, recycled, and engineered into countless forms around us.
Aluminium’s unique combination of lightweight strength, corrosion resistance, and excellent thermal conductivity makes it a favorite material in everything from airplanes and cars to smartphones and kitchenware. But behind all these applications lies a fundamental property: the temperature at which aluminium transitions from a solid to a liquid. This single characteristic influences how aluminium is:
Imagine designing an automotive engine component or a lightweight bicycle frame. If you don’t understand the melting behavior of aluminium, you risk creating products that might deform under heat, fail during welding, or suffer from casting defects. Even everyday items like foil or beverage cans rely on precise knowledge of aluminium’s melting properties to guarantee quality and safety.
This article will guide you through all the essential facts and key insights about aluminium melt temperature. We’ll start by defining what a melting point is and why it’s vital for metals. Next, you’ll discover the scientifically accepted melting temperature for pure aluminium and learn how factors like alloying, impurities, and pressure can influence this property. We’ll also explore how different aluminium alloys behave, compare aluminium’s melting point to other metals, and discuss real-world implications for manufacturing, recycling, and product design.
By the end of this guide, you’ll not only know what temperature aluminium melts at but also understand why this knowledge is a cornerstone of modern engineering and manufacturing success. Ready to dive in? Let’s start with the basics of what a melting point means for metals.
When you heat a metal, have you ever wondered exactly what happens as it turns from solid to liquid? The answer lies in a fundamental concept called the melting point of metals. Let’s break it down so it’s easy to understand—even if you’re not a scientist.
The melting point is the precise phase transition temperature at which a solid material changes into a liquid under standard atmospheric pressure. At this temperature, both the solid and liquid forms of the metal coexist in equilibrium—think of an ice cube just beginning to melt on a warm day. For metals, this is a defining property that helps determine how and where they can be used in manufacturing and engineering (Polyspectra).
So, what’s really happening at the atomic level? Metals are held together by strong metallic bonds—think of them as a sea of electrons gluing the atoms in place. To melt a metal, energy (in the form of heat) must be supplied to overcome these bonds. The stronger the bonds, the higher the energy required, and therefore, the higher the melting point. This is why metals like tungsten have extremely high melting points, while others like aluminum melt at much lower temperatures.
In short, the melting point of metals is more than just a number—it’s a critical threshold that shapes how we use, process, and trust metals in everything from skyscrapers to smartphones. Next, let’s look at the exact melting temperature for pure aluminum and why this benchmark matters across industries.
When you ask, "what is the melting temperature of aluminium?", the answer is both precise and foundational for countless industrial processes. Pure aluminum—defined as aluminum with a purity of 99.9% or higher—has a scientifically accepted melting temperature of 660.32°C (1220.58°F). This value is not just a trivia fact; it’s a crucial benchmark that underpins everything from casting car parts to recycling beverage cans.
This aluminium melting temperature is measured under standard atmospheric pressure using precise laboratory equipment such as calibrated thermocouples and controlled heating environments. By heating a pure aluminum sample and monitoring the temperature at which it transitions from solid to liquid, scientists establish this reliable point of reference. The accuracy is vital: even a small deviation can impact product quality or safety in applications where temperature control is critical.
Imagine casting an engine block or designing a heat exchanger. Without a clear understanding of the aluminium melting temperature, you risk defects, energy waste, or even catastrophic failure. That’s why this value is more than just a number—it’s the cornerstone of aluminum’s role in modern manufacturing. In the next section, we’ll explore what happens when alloying elements or impurities come into play, and how they can shift this critical temperature.
When you’re working with aluminum—whether casting, welding, or selecting materials for a new product design—you might wonder, why doesn’t every piece of aluminum melt at exactly the same temperature? The answer lies in a handful of critical factors that can shift or even broaden the melting behavior of this versatile metal. Let’s break down the main aluminium melting point factors so you can make informed decisions for your next project.
Imagine pure aluminum as a clean slate, melting consistently at around 660°C (1220°F). But in real-world applications, aluminum is rarely used in its pure form. Instead, it’s often combined with other metals to create alloys that enhance specific properties. Here’s how common alloying elements affect the aluminium alloy composition and its melting point:
Because these elements don’t just shift the melting point, but often create a melting range (with separate solidus and liquidus temperatures), understanding the exact aluminium alloy composition is essential for precise process control. This is especially true in critical industries like aerospace and automotive, where even small variations can impact safety or performance.
Even trace amounts of unintended elements—known as impurities—can influence the melting characteristics of aluminum. Here’s what you’ll notice:
To ensure consistent results, manufacturers often use refining and filtration techniques to minimize the impact of impurities. This is especially important for applications demanding tight tolerances or superior mechanical properties.
Pressure can also affect the melting point, but under normal atmospheric conditions, its impact is minor. If you’re working in specialized environments—like high-pressure casting or vacuum systems—you might observe slight increases or decreases in the melting temperature. For most manufacturing and fabrication scenarios, however, pressure isn’t a significant concern.
With so many variables at play, how do you ensure your aluminum profiles or components will perform as needed? Sourcing alloys with precise compositions and controlled impurity levels is key. Reputable manufacturers, such as Shengxin Aluminum, specialize in delivering consistent, high-performance profiles tailored to the demands of industries like transportation, construction, and electronics. Their expertise in alloy selection and process control helps guarantee that your materials will meet both technical and safety standards—no surprises, just reliable results.
Understanding these melting point factors empowers you to select the right aluminum for every application. Up next, we’ll dive into the specific melting behavior of aluminum foil and why it remains stable in your kitchen, even when exposed to high oven temperatures.
Ever wondered why your aluminum foil stays perfectly intact in the oven, even when you crank up the temperature for a crispy roast or a gooey batch of cookies? The answer lies in the unique properties of aluminum foil—and understanding its aluminium foil melting temperature can help you use it with confidence in everyday cooking and baking.
Let’s get right to the numbers: the aluminium foil melting temperature is approximately 660°C (1220°F). This is nearly identical to pure aluminum because most household foil is made from highly refined aluminum, typically over 98% pure (Southern Living). Despite its ultra-thin form—sometimes less than 0.02 millimeters thick—foil retains the same fundamental melting point as bulk aluminum.
Here’s a practical scenario: your oven at home typically reaches a maximum of 500°F to 550°F (about 260°C to 290°C), far below the aluminium foil melting temperature. Even commercial ovens rarely exceed 600°F (315°C). That means, under normal cooking or baking conditions, aluminum foil is nowhere near its melting point and remains structurally stable.
So, whether you’re lining baking trays, wrapping leftovers, or shielding pie crusts from over-browning, you can trust that aluminum foil will hold up—no matter how hot your oven gets for typical recipes. Just remember: while foil is tough, it’s not invincible. Avoid direct contact with open flames or broilers at extremely high temperatures, and always use foil according to manufacturer guidelines for best results.
Now that you know why aluminum foil remains steadfast in your kitchen, let’s turn our attention to how aluminum alloys behave differently—often melting over a range of temperatures rather than at a single point.
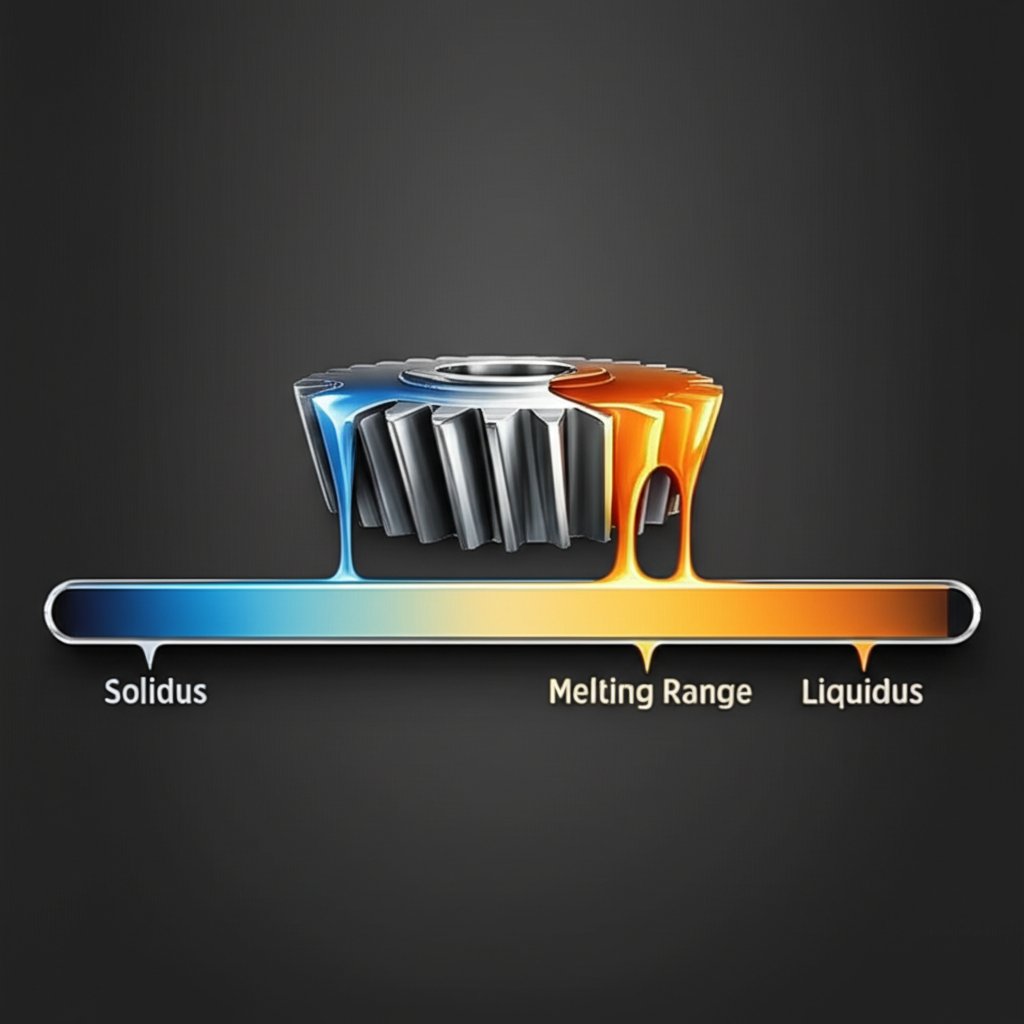
When you work with aluminum alloys, you’ll quickly notice that they don’t melt at a single, sharp temperature like pure aluminum does. Instead, most alloys have what’s called a melting range. Sounds complex? Let’s break it down with simple terms and real-life examples, so you’ll never be caught off guard at the foundry, in the workshop, or during a design review.
Unlike pure aluminum, which transitions from solid to liquid at 660.32°C (1220.58°F), aluminum alloys are made by combining aluminum with other elements—like silicon, copper, magnesium, or zinc. These additions are great for boosting strength, corrosion resistance, or machinability, but they also change the way the alloy melts. Instead of a single temperature, you get a range between two key points: solidus and liquidus.
This melting range is a defining characteristic of the aluminium alloy melting temperature and is crucial for controlling manufacturing processes.
Imagine you’re casting a complex part or welding two critical components. If you only consider the liquidus temperature, you might overheat the alloy, causing excessive fluidity, shrinkage, or even unwanted reactions. On the other hand, if you stay too close to the solidus, you risk incomplete melting, leading to poor fill, cold shuts, or weak welds.
Let’s make this even clearer. Here’s a table that illustrates solidus, liquidus, and the melting range for a generic aluminum alloy. These values are for demonstration—actual numbers will vary by alloy composition:
| Alloy Type | Solidus (°C) | Liquidus (°C) | Melting Range (°C) |
|---|---|---|---|
| Generic Aluminum Alloy | 570 | 650 | 80 |
So, if you’re working with an alloy that starts to melt at 570°C and becomes fully liquid at 650°C, you know there’s an 80-degree window where the metal is partially melted. This is the "sweet spot" for certain manufacturing processes, but it also demands careful temperature control.
Understanding the melting temperature of aluminium alloys—and more specifically, their melting ranges—helps you avoid costly errors, improve product quality, and choose the right alloy for the job. Whether you’re casting, welding, or designing, always check the solidus and liquidus values for your specific alloy. Up next, we’ll zoom in on some of the most popular aluminum alloys, like 6061 and 6082, and see how their melting ranges influence how they’re used in industry.
When you’re deciding which aluminum alloy to use for a project—whether it’s a bridge, a bike frame, or a piece of precision machinery—you’ll often encounter 6061 and 6082. But what makes these alloys so popular, and how do their melting characteristics affect how they’re processed? Let’s dive into the details, breaking down their melting ranges and real-world uses, so you can make the right choice for your next engineering challenge.
Unlike pure aluminum, which melts at a single temperature, both 6061 and 6082 have a melting range—a span between the solidus (start of melting) and liquidus (fully melted) temperatures. Knowing these values is crucial for casting, welding, and heat treatment, because it allows you to control the process and avoid defects.
| Alloy | Solidus (°C) | Liquidus (°C) | Melting Range (°C) |
|---|---|---|---|
| 6061 | 582 | 652 | 70 |
| 6082 | Approx. 585 | Approx. 652 | ~67 |
6082 values based on industry norms and alloy family characteristics (Wikipedia).
Imagine you’re welding a bike frame or extruding profiles for a high-speed train. If you don’t respect the melting range, you could end up with weak joints, poor surface finish, or even catastrophic failure. Here’s how the melting behavior shapes their use:
When your project demands consistent, high-performance aluminum profiles—whether for transportation, construction, or precision engineering—it pays to work with a manufacturer who understands the nuances of alloy melting ranges. Shengxin Aluminum leverages advanced production capabilities and deep expertise to deliver precisely engineered 6061 and 6082 profiles. Their commitment to quality and process control means you get components that meet the toughest industry standards, every time.
As you consider which alloy fits your needs, keep in mind how melting behavior impacts everything from manufacturability to long-term performance. Up next, we’ll see how aluminum’s melt temperature compares to other metals and materials, helping you understand its unique position in the world of engineering.

Ever wondered how aluminum stacks up against other metals and materials when it comes to heat resistance? Whether you’re designing a high-temperature component or just curious about why some metals melt faster than others, understanding these differences can help you make smarter choices in engineering and manufacturing.
Let’s start with a practical scenario: imagine you’re selecting materials for a part that must withstand high heat. You might ask, “Is aluminum more heat-resistant than copper or steel? What about non-metals like glass or aluminum oxide?” To answer these questions, let’s look at the melting temperatures of some widely used metals and materials, including the melting temperature of copper and aluminium and others:
| Material | Melting Point (°C) | Melting Point (°F) |
|---|---|---|
| Lead | 328 | 621 |
| Zinc | 420 | 787 |
| Magnesium | 650 | 1200 |
| Aluminum (Pure) | 660 | 1220 |
| Copper | 1084 | 1983 |
| Stainless Steel | 1510 | 2750 |
| Carbon Steel | 1371–1593 | 2500–2900 |
| Aluminum Oxide (Al2O3) | 2072 | 3762 |
| Soda-Lime Glass | 1400–1600 | 2552–2912 |
| Borosilicate Glass | 820–1100 | 1508–2012 |
Understanding how aluminium melt temperature compares to other substances helps you select the right material for every job—balancing process temperatures, safety, and performance. Next, let’s see how these melting points play out in real-world manufacturing, casting, and welding applications.
When you think about the temperature for aluminium melt, it might seem like a detail for the lab. But in reality, this number shapes everything from how car parts are cast to how your soda can is recycled. Curious how? Let’s walk through the real-world scenarios where the aluminium melt temperature isn’t just a fact—it’s the foundation for safe, efficient, and high-quality manufacturing.
Imagine a busy foundry floor: glowing furnaces, molten metal, and precise timing. To cast aluminum parts, you can’t just heat to the melting point and call it a day. Here’s how it works in practice:
Ever tried to weld aluminum and found it trickier than steel? That’s because aluminum’s high thermal conductivity and low melting point demand precision:
Picture aluminum being squeezed through a die to create window frames or bike tubes. The trick? Heating the billet just below its melting range:
Want to boost aluminum’s strength or ductility? Heat treatment is the answer—but it’s all about precision:
Handling molten aluminum is no small feat. It’s hot, reactive, and can be hazardous if not managed properly:
Understanding the aluminium melting furnace temperature and related process nuances isn’t just for engineers—it’s for anyone who values quality, efficiency, and above all, safety. As you move forward, remember that every application—casting, welding, extrusion, or heat treatment—relies on a deep respect for aluminum’s melting behavior. Next, let’s wrap up with the essential facts and a summary of why mastering melt temperature knowledge is key to innovation and reliability in modern manufacturing.
When you step back and look at the big picture, it’s clear that understanding aluminium melt temperature is more than a technical detail—it’s a foundation for success in modern manufacturing, engineering, and product design. From the precise melting point of pure aluminum (660.32°C/1220.58°F) to the nuanced melting ranges of popular alloys like 6061 and 6082, every fact you’ve learned here helps unlock new levels of safety, efficiency, and creative potential.
Imagine launching a new product or optimizing a manufacturing line. If you understand both the fundamental and alloy-specific melting behaviors, you’ll:
In short, mastery of aluminium melt temperature isn’t just for metallurgists—it’s a competitive advantage for anyone shaping the future of design and manufacturing.
If your project demands high quality aluminium profiles engineered for specific melting and processing requirements, the choice of manufacturer is critical. Partnering with experts like Shengxin Aluminum ensures you benefit from advanced alloy selection, precise process control, and a commitment to excellence at every stage. Their experience in delivering custom, high-performance profiles means your solutions are always reliable, innovative, and tailored to your exact needs.
Ready to elevate your next project? Consult Shengxin Aluminum for profiles that set the standard in quality and performance.
Aluminum foil can withstand temperatures up to approximately 660°C (1220°F) before melting. Typical household ovens only reach about 260°C (500°F), so aluminum foil remains stable and safe during regular cooking and baking tasks.
Yes, aluminium is considered easy to melt compared to many other metals. Its relatively low melting point means it can be melted with a hand-held torch or standard furnace, making it ideal for recycling, casting, and manufacturing processes.
Pure aluminium melts at 660°C (1220°F), so it will be fully melted at 750°C. However, most aluminium alloys may begin to lose strength at lower temperatures, so it’s important to know the specific alloy’s melting range for proper processing.
Most aluminium alloys contain additional elements like silicon, copper, or magnesium. These create a melting range, defined by solidus (start of melting) and liquidus (fully melted) temperatures, rather than a single melting point. This range is crucial for processes like casting and welding.
Shengxin Aluminum is a leading manufacturer with advanced production capabilities, strict quality control, and expertise in producing high-performance aluminium profiles for demanding industries such as transportation and construction. Their focus on precise alloy composition ensures consistent melting behavior and reliable product performance.
 Интернет Сервис
Интернет Сервис 0086 136 3563 2360
0086 136 3563 2360 sales@sxalu.com
sales@sxalu.com +86 136 3563 2360
+86 136 3563 2360